Researchers Strive to Make Thermoset Polymers Easier to Recycle
UK team develops way to introduce degradable bonds into thermosets such as rubbers, gels, and adhesives
Thermoset plastics are incredibly strong and flexible, owing to their covalently crosslinked polymer chains. Such materials, as well as other crosslinked polymers such as elastomers and polymer gels, can exhibit exceptional mechanical and thermal properties. But the very molecular structure that gives them strength also makes them challenging to recycle.
Unlike thermoplastics, they cannot simply be reheated and reshaped. Rather, the crosslinks in thermosets mean the materials burn rather than melt when heated, making them much harder to break down.
Introducing degradable bonds to enhance thermoset recycle

Maciej Kopeć, senior lecturer at the University of Bath
Researchers in the U.K., however, now say they have developed a way of introducing degradable bonds into thermoset polymers to make them easier to recycle. These scientists, at the University of Bath and the University of Surrey, published their findings in a recent issue of Polymer Chemistry detailing their use of cleavable/reversible bond chemistry.
Technically speaking, they said they synthesized degradable poly(n-butyl acrylate) networks by reversible addition–fragmentation chain transfer (RAFT) polymerization using a cleavable disulfide diacrylate crosslinker or a cleavable comonomer, dibenzo[c,e]oxepine-5(7H)-thione (DOT).
The researchers — Frances Dawson and Maciej Kopeć from the University of Bath, and Touseef Kazmi and Peter J. Roth from the University of Surrey — wrote: “When designing a degradable polymer network, the aim is to install degradable units into the network without compromising the material’s mechanical properties.” They said they achieved this goal by analyzing gelation kinetics, equilibrium swelling ratio, and storage modulus.
Reversible bond chemistry
They successfully synthesized reversible poly(n-butyl acylate) networks by RAFT polymerization using either a disulfide crosslinker or a degradable comonomer. Whereas disulfide crosslinkers are ineffective at making networks fully degradable when synthesized by conventional free radical polymerization (FRP), they explained, that the use of RAFT polymerization affords degradability networks at a range of crosslinking densities.
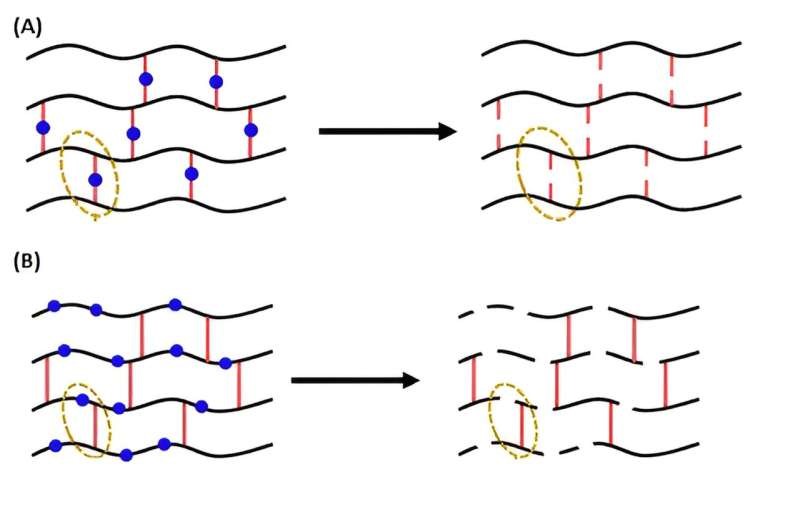
In addition to cleavable/reversible bond chemistry, they added, that the location of these degradable units within the network also significantly affects their degradability. For example, such units can be installed either in the primary chains (strands) of the polymer network or within the crosslinks.
The team said they made a series of polymer gels with breakable bonds incorporated into different parts of the structure. They then tested to determine if the properties changed after the gel was degraded and reformed.
All the gels could be degraded to some extent, they found, but compared with the polymers that were broken down via the cross-linked bonds, the gels they created with breakable bonds retained their properties much better when reformed.
Considering network topology
“The influence of network topology becomes even more pronounced for gels synthesized by free radical polymerization. In principle, using a crosslinker with a labile bond incorporated into it should be a simple method of making a degradable network. However, both polymerization technique and monomer choice can affect the degradability of the resulting networks.”
On the other hand, they continued, networks made by reversible deactivation radical polymerization (RDRP) techniques “have a more homogenous structure due to the mechanism of controlled chain growth without formation of microgel clusters.”
Beyond recyclable thermosets, degradable and/or reversible crosslinks also can enable many advanced applications of polymer networks, such as drug delivery systems, or self-healing materials.
The UK’s Engineering and Physical Sciences Research Council (EPSRC) is funding their research.
