Polymer Sorbents for Critical Minerals

Engineered polymers extract rare-earth ions from contaminated leachates, reducing acid consumption, cycle time, and solvent inventories.
Lithium, nickel, cobalt, and rare earth elements underpin batteries, generators, and electronics that power the energy transition. However, global demand continues to rise steeply, while conventional mining remains constrained by long permitting cycles, high water consumption, and extensive land disturbance. Although hydrometallurgy improves recovery efficiency, it still depends on strong acids and generates corrosive effluents.
You can also read: NAFION: Production, Advantages, and Future Challenges.
In contrast, polymer science now provides a direct molecular route—capturing ions from brines, mine effluents, geothermal fluids, and black-mass leachates through engineered sorbents.
Moreover, this shift is not theoretical—it is already underway. Pilot lines in Japan, Germany, and the United States are producing cross-linked beads, asymmetric membranes, and fiber mats that selectively bind lithium and rare-earth cations from feeds measured in mere parts per million. As a result, these systems reduce tanker shipments, minimize solvent inventories, and significantly lower energy intensity.
Ultimately, extrusion, functional monomer design, column engineering, and regeneration chemistry converge in a single, integrated process, transforming plastics into active agents for critical mineral recovery rather than passive packaging materials.
How Polymer Sorbents Capture Ions
At their core, polymer sorbents incorporate ion-selective ligands. Phosphonic and carboxylic groups chelate rare-earth ions such as Nd³⁺, Dy³⁺, and Pr³⁺, while crown-ether–modified acrylates coordinate lithium through precise cavity dimensions. Furthermore, advanced platforms now include channel-mimetic architectures and porous hydrogels grafted with chelators. Engineers fine-tune pore-size distribution, crosslink density, and hydrophilicity to optimize both mechanical integrity and diffusion kinetics. During operation, plants follow a cyclic process: they pass brine through packed beds or hollow-fiber modules, load the target ions until breakthrough, and then elute with a mild acid or base to restore capacity. Nevertheless, challenges persist. Competing ions such as Mg²⁺ and Ca²⁺ interfere with selectivity, while dissolved oxygen oxidizes ligands and fouling agents—silica, hydrocarbons, and iron oxides—clog pores.
Therefore, operators counteract these effects through staged filtration, antiscalant dosing, and redox control, followed by defined washing and regeneration protocols.
Lithium from Brine, Geothermal Water, and Batteries
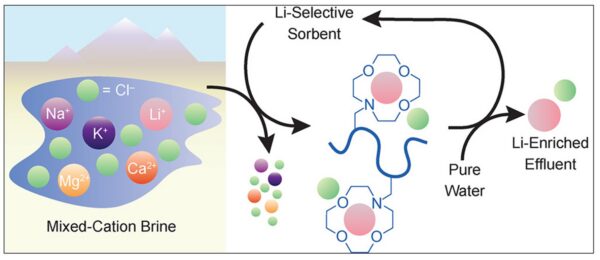
Polymer sorbents selectively bind lithium ions from multicomponent brines, producing a Li-enriched stream with minimal water and chemical use. Courtesy of Microporous Polymer Sorbents for Direct Lithium Extraction.
Polymer sorbents are now enabling lithium extraction from low-concentration brines in Chile, Argentina, and Bolivia. Whereas traditional solar evaporation takes up to two years and consumes vast water volumes, polymer sorbents reduce this to mere hours. They achieve selective lithium capture with minimal reagent use by employing manganese-oxide–coated or phosphoryl-functionalized matrices. In addition, geothermal lithium projects integrate these polymers directly into production lines. For instance, in Germany, hot brine passes through lithium-selective polymer beds located downstream of geothermal turbines. The brine is then re-injected underground, while the lithium-enriched eluate proceeds to recovery. Similarly, California’s Salton Sea pilot plants use polymer-bound ion sieves that tolerate both high salinity and silica content, ensuring stable operation under extreme chemical and thermal conditions.
Rare Earth Recovery from Waste Streams

Electronic waste contains high-value metals like lithium, cobalt, and rare earths. Courtesy of Orissa International.
The recovery of rare earth elements (REEs) from coal waste, e-waste, and magnet scrap offers another major frontier. Because conventional REE extraction produces radioactive residues and acidic tailings, polymer-based approaches provide a cleaner alternative. Plants now deploy IDA-functionalized polymer beads in fluidized beds to treat leachates, operating within tight pH windows to maintain selectivity while minimizing co-extraction of Fe(III) and Al(III). Inline sensors continuously track breakthrough profiles and differential pressure, maintaining stable separation efficiency over many cycles. Meanwhile, membrane sorbents advance on two paths. Supported liquid membranes (SLMs) deliver excellent selectivity but suffer from pore wetting and carrier loss.
Consequently, research now pivots toward polymer inclusion membranes (PIMs), which immobilize extractants directly in solid supports. PIMs merge extraction and stripping in one step, eliminate carrier leaching, and maintain mechanical flexibility—particularly favoring heavy rare-earth elements.
Processing and Scale-Up: Polymer Expertise at Work
Sorbent fabrication begins with familiar base polymers such as polyethylene, polypropylene, polystyrene, or polyacrylonitrile. Processors extrude or spin fibers, mold monoliths, and graft functional ligands through radiation, plasma, or reactive extrusion.
Among these, reactive extrusion stands out for its continuous operation and cost efficiency. Engineers feed maleic anhydride, glycidyl methacrylate, or vinyl phosphonic acid into twin-screw extruders with precise residence-time control. Afterward, washing and neutralization remove unreacted monomers, producing pellets or films ready for column or membrane assembly.
However, durability determines commercial success. Sorbents must withstand thousands of adsorption–desorption cycles. To achieve this, developers copolymerize elastomeric segments to improve toughness and resist crack propagation. Others add porous silica shells to minimize abrasion while maintaining mass transfer, and introduce crosslinkers that stabilize ligand–polymer bonds during repeated acid/base exposure.
Opportunities for the Plastics Industry
The rise of polymer sorbents opens a new interdisciplinary frontier for the plastics sector. Success demands not only polymer formulators, extruder operators, and mold designers, but also experts in water chemistry, life-cycle assessment, and process control.
Key challenges remain: scalable membranes that resist fouling for months, sorbents maintaining over 90% capacity after 1,000 cycles, and affordable functional monomers compatible with existing infrastructure. Moreover, sustainable end-of-life strategies for spent sorbents must evolve alongside integration with desalination, geothermal, and battery-recycling systems.
Ultimately, this convergence of polymer science and resource recovery represents more than a technical breakthrough—it is a blueprint for circularity. By transforming plastic from waste into a tool for extraction, the industry can reclaim both materials and purpose.
