Hydrogels in Drug Delivery: Smart Carriers for Targeted Therapies

Hydrogels offer smart drug delivery by mimicking tissue, holding water, and targeting disease sites with controlled therapeutic release.
Hydrogels are emerging as a transformative technology in drug delivery, providing solutions to some of the most pressing challenges in modern medicine. Their three-dimensional polymer network, high water retention, and soft tissue-mimicking properties make them ideal for carrying therapeutic agents directly to disease sites. Whether in gels, sheets, nanoparticles, or injectable formulations, hydrogels offer customizable platforms that can release controlled, sustained, and targeted drugs. These characteristics are especially valuable in treating chronic illnesses, cancer, and wounds where precision and reduced toxicity are paramount.
You can also read: Polymeric Biosensors: A New Era in Medical Diagnostics
Precision Drug Delivery with Hydrogels
One of the most significant advantages of hydrogels is their ability to encapsulate drugs and release them over time, either passively or in response to specific triggers. This minimizes the need for frequent dosing and reduces systemic exposure, which are key factors in improving patient compliance and outcomes. Researchers can engineer hydrogels to respond to external stimuli like temperature or light and internal cues such as pH or enzyme activity. These stimuli-responsive systems enable site-specific and on-demand drug release, a feature highly desirable in sensitive treatments like chemotherapy or post-surgical healing.
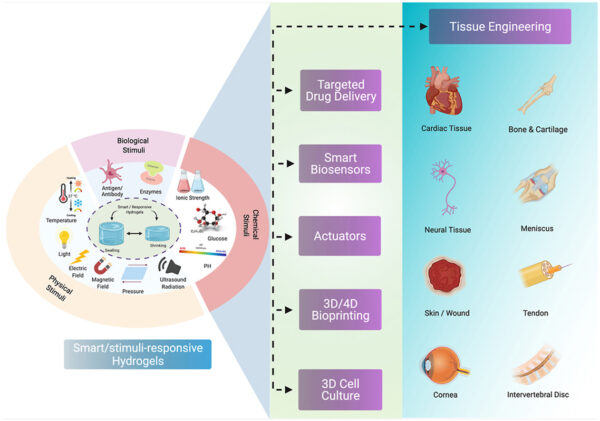
Schematic illustration of different smart/stimuli-responsive hydrogels employed for different biomedical applications. Courtesy of Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications.
Customizable Structures for Controlled Release
One of hydrogels’ greatest strengths is their structural versatility. By altering the cross-linking density, polymer composition, and degradation rate, scientists can fine-tune the drug’s release kinetics. This allows precise control over how quickly a drug is released, how long it remains active, and where it exerts its effect. Hydrogels can be synthesized from natural polymers like alginate, gelatin, or chitosan, known for their biocompatibility and biodegradability, or from synthetic polymers like polyethylene glycol (PEG) and polyvinyl alcohol (PVA), which offer mechanical strength and chemical stability. Recent advances have led to composite hydrogels, which combine the best of both worlds, and nanogels, which offer even more refined control at the nanoscale.
Clinical Applications: From Tumors to Tissue Repair
Hydrogels are being tested and used in a wide array of clinical applications. In oncology, hydrogels serve as vehicles for chemotherapeutic drugs, allowing their localized release at tumor sites, which reduces collateral damage to healthy tissues and decreases adverse effects. In wound healing, hydrogel dressings not only keep the wound moist—a key requirement for tissue regeneration—but also serve as carriers for antimicrobials, anti-inflammatories, and growth factors. These hydrogels accelerate healing while preventing infection. Other promising areas include ocular drug delivery, transdermal systems, and central nervous system therapies, where the sensitive nature of the tissue demands minimally invasive, targeted, and biodegradable delivery mechanisms.
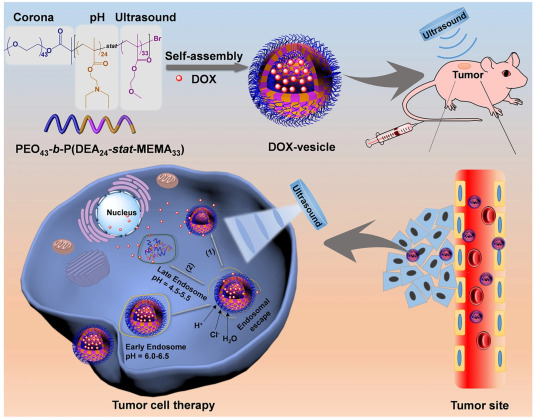
Schematic illustration of ultrasound-sensitive targeted Doxorubicin drug delivery in tumor cells. Copyright © 2020, ELSEVIER Publishing Group. Courtesy of Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications.
Comparison of Hydrogel Drug Delivery Types
| Hydrogel Type | Polymer Source | Stimuli Response | Applications | Advantages |
|---|---|---|---|---|
| Natural (e.g., alginate, chitosan) | Biological (plant, animal) | pH, enzymes | Wound healing, tissue repair | Biodegradable, low toxicity |
| Synthetic (e.g., PEG, PVA) | Chemical synthesis | Temperature, light | Cancer therapy, long-term dosing | Tunable properties, mechanical strength |
| Composite (e.g., micelle–hydrogel) | Natural + synthetic | Multiple stimuli | Smart release systems | Multi-functionality, improved targeting |
| Nanogels | Nano-sized hydrogels | pH, heat | Ocular, transdermal delivery | High surface area, rapid response |
| Injectable hydrogels | In situ-forming gels | Temperature, ionic strength | Minimally invasive delivery | Easy administration, patient comfort |
Looking Ahead
As the demand for personalized and precision medicine grows, hydrogel-based delivery systems will continue to evolve. Researchers are exploring multi-drug loading, responsive release cascades, and bioactive hydrogel scaffolds that not only deliver drugs but also support tissue regeneration or immune modulation. Hydrogels stand at the intersection of materials science, pharmacology, and biomedical engineering—making them a cornerstone in the future of safe, targeted, and efficient drug delivery.
