Zinc Dodecyl Sulfate: A Cleaner Path to Rubber Vulcanization

Zinc Dodecyl Sulfate boosts rubber strength and stiffness while reducing zinc waste, offering a greener alternative to ZnO in Styrene-butadiene rubber vulcanization.
The tire and rubber industries are under growing pressure to reduce environmental pollution without compromising performance. One key area of concern is zinc oxide (ZnO), which is traditionally used in rubber vulcanization but is increasingly scrutinized due to zinc leaching into ecosystems. A novel approach developed by Tavakoli et al. (2025) introduces zinc dodecyl sulfate (Zn(DS)₂) as a partial substitute for ZnO in sulfur-cured styrene-butadiene rubber (SBR). This new zinc complex significantly reduces unreacted zinc content while enhancing key mechanical properties.
You can also read: Tire Recycling: Feasibility and Profitability.
Reduced Zinc Pollution with Zinc Dodecyl Sulfate
A major achievement of this study is the reduction in unreacted zinc. As shown in the table, by substituting ZnO with increasing amounts of Zn(DS)₂ led to up to 77.1% less unreacted zinc:
| Sample | Unreacted Zn (mg/L) | % Reduction |
|---|---|---|
| C0Z3 | 0.35 | 0% |
| C1.5Z1.5 | 0.13 | 62.8% |
| C2Z1 | 0.08 | 77.1% |
This outcome indicates higher zinc ion availability from Zn(DS)₂, which promotes more efficient interaction with accelerators and reduces waste.
Improved Mechanical Properties
Incorporating zinc dodecyl sulfate (Zn(DS)₂) into the rubber compounds led to a notable increase in tensile strength compared to the conventional ZnO formulation. The highest tensile strength was observed in the compound containing equal ZnO and Zn(DS)₂ (C1.5Z1.5). Beyond this ratio, further increases in Zn(DS)₂ caused a decline in strength, likely due to excessive crosslinking and reduced chain mobility.
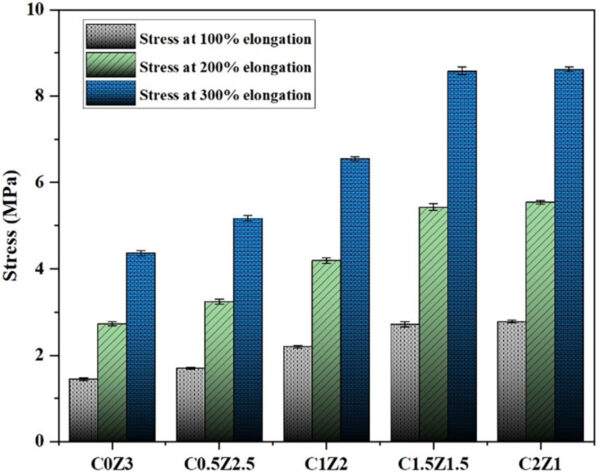
Stress at 100%, 200%, and 300% elongation of samples. Courtesy of A novel zinc complex for rubber vulcanization: Enhanced mechanical properties and reduced environmental impact through ZnO substitution.
As tensile strength increased, elongation at break decreased progressively with higher Zn(DS)₂ content. This inverse relationship indicates that although the material became stronger, it became less flexible. Moreover, stress at 100%, 200%, and 300% elongation increased consistently with the addition of Zn(DS)₂, reflecting higher stiffness and resistance to deformation. Similarly, hardness values rose across all samples containing Zn(DS)₂, correlating with the elevated crosslink density induced by the complex.
Overall, Zn(DS)₂ enhances the mechanical performance of the vulcanized rubber by increasing strength, modulus, and hardness, although it reduces extensibility at higher concentrations.
Enhanced Vulcanization Efficiency
Zn(DS)₂ accelerates vulcanization by reducing scorch time (ts₂), thanks to its compatibility with the hydrophobic rubber matrix. As shown, the scorch time dropped from 6:06 to 3:24 (min:sec) as Zn(DS)₂ increased.
| Sample | Scorch Time (ts₂) | Optimum Cure Time (t₉₀) |
|---|---|---|
| C0Z3 | 6:06 | 17:56 |
| C2Z1 | 3:24 | 28:08 |
This shorter onset time ensures faster processing, although the extended cure time (t₉₀) suggests prolonged crosslinking, likely due to stronger Zn-ligand coordination.
Dynamic and Thermal Behavior
The inclusion of Zn(DS)₂ also improved dynamic mechanical performance. As seen in Figure 9, rolling resistance (tan δ at 60°C) was reduced, particularly in C0.5Z2.5, which supports better fuel efficiency in tires. However, thermal stability slightly declined with Zn(DS)₂, as TGA results (Figure 10) showed a modest drop in degradation onset temperature. This is attributed to the organic nature of the Zn(DS)₂ ligand.
By partially replacing ZnO with Zn(DS)₂, the researchers achieved a sustainable, high-performance rubber compound. The novel activator boosts tensile properties and crosslink density while dramatically cutting zinc emissions, making it a promising green innovation for the tire industry.
