Innovative Wound Care Device Relies on High-Tech Polycarbonate
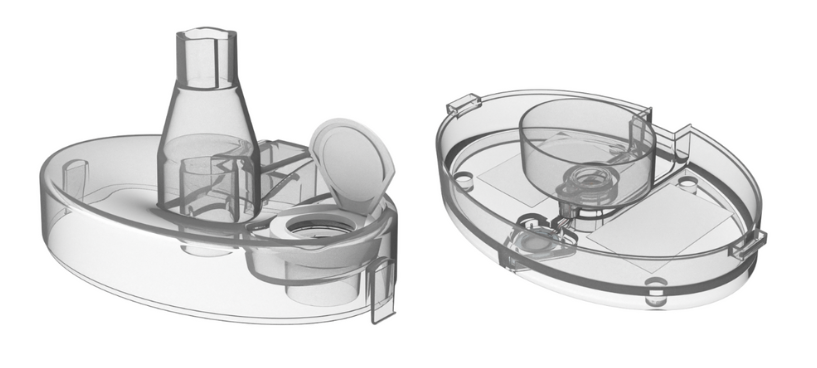
The company Vaporox created a device with low-frequency ultrasound technology that treats non-responsive chronic wounds, using a high-tech polycarbonate.
Specialized equipment that enhances skin recovery in chronic wounds usually needs biocompatible, sterilizable, and durable materials. For this reason, Vaporox developed the VHT-200 to foster revascularization and tissue growth in chronic skin wounds using a high-tech polycarbonate. Tailored for clinic and physician office use, the medical device required a robust material for its vaporizer component. Due to the device’s significance, technical support from the plastic supplier was a crucial factor in the decision-making process.
Find robust material for a life-changing medical device
For this device, Covestro supplied Vaporox with its Makrolon® 2458 polycarbonate, a low-viscosity, injection-molding medical grade. This polycarbonate is extremely robust, lightweight with glass-like transparency, and is impact resistant – even at low temperatures. It also has a high dimensional stability and is easily molded, yet displays excellent heat resistance with a glass transition temperature of up to 148ºC. In Addition, it can be used in general purpose, lighting, medical and food contact, flame-retardant, impact-modified and glass-fiber reinforced grades,
Key Benefits
- Sterilizable Suitable for Ethylene Oxide (EtO) and steam sterilization
- Biocompatible Meets several biocompatibility criteria
- Easy to process Low-viscosity, injection-molding medical grade features easy release from the mold
- Design freedom for tough applications, yet attractive.
“For decades, the healthcare market has relied on our proven portfolio of medical-grade Makrolon polycarbonate. Our work with Vaporox demonstrates the value of collaboration as our customers develop and bring truly innovative, game-changing technology to market.” Said Mark Nichols, Healthcare Key Account Manager at Covestro LLC.
These key features and the partnering between Vaporox and Covestro have allowed them to change many people’s lives. In effect, this innovative medical device received U.S. Food and Drug Administration clearance in 2023.
