Cast21’s Innovative Resin Casts for Improved Healing

Transforming Patient Experience with Advanced Materials
Jason Troutner, Chief Technology Officer of Cast21, knows a thing or two about casts—having spent over three years of his life in more than 50 fiberglass casts due to surgeries and sports injuries. Joined by biomedical design engineer Ashley Moy and electrical engineer Justin Brooks, Troutner co-founded Cast21 to revolutionize the discomforts associated with traditional casts.
Traditional fiberglass casts, though an improvement over plaster ones, have their drawbacks. They aren’t waterproof, leading to water absorption and unpleasant odors. Moreover, wearers still need to protect the cotton padding beneath from getting wet. Recognizing these issues, the Cast21 team set out to create a patient-friendly solution.
Innovative Resin Casts: Waterproof and Breathable
Cast21’s innovative approach involves a cast made from interconnected silicone tubes that harden when injected with resin. Unlike conventional casts, its lattice design immobilizes bones while leaving most of the skin exposed, promoting breathability. This not only addresses comfort concerns but also opens avenues for additional therapies.
The new cast can be used alongside electrical stimulation therapy, reducing healing time. The silicone material allows for the integration of electrical stimulation electrodes, aiding in preventing muscle atrophy and supporting fracture healing.
Recent Breakthrough: 3D-Printed Exoskeleton
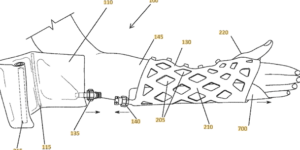 On October 5, 2023, Cast21’s innovative PCT application was published by the World Intellectual Property Organization, unveiling its revolutionary 3D-printed exoskeleton—a transformative alternative to traditional casts. Crafted from a medical-grade resin, this orthopedic solution addresses common cast issues, such as water-related problems leading to infections and reduced support. Cast21’s FDA-approved product, highlighted in their white paper, boasts a waterproof and breathable design, enabling patients to engage in activities like showering, swimming, or washing their limbs without compromising the cast’s integrity.
On October 5, 2023, Cast21’s innovative PCT application was published by the World Intellectual Property Organization, unveiling its revolutionary 3D-printed exoskeleton—a transformative alternative to traditional casts. Crafted from a medical-grade resin, this orthopedic solution addresses common cast issues, such as water-related problems leading to infections and reduced support. Cast21’s FDA-approved product, highlighted in their white paper, boasts a waterproof and breathable design, enabling patients to engage in activities like showering, swimming, or washing their limbs without compromising the cast’s integrity.
An earlier patent granted in June 2022 details the incorporation of a “lattice structure” in the Cast21 product. This involves injecting a “liquid resin and a catalyst mixture that transforms into a solid” into the lattice structure. The recently published patent application in October 2023 extends the coverage, seeking additional protection for various cast configurations and complementary accessories. This marks a significant advancement in orthopedic care, where the fusion of resin technology and 3D printing redefines the landscape of cast solutions.
The Path Forward
Collaborating with medical device accelerator ZeroTo510, the team aims to establish a robust sales and marketing structure. Meanwhile, Troutner is fine-tuning the silicone and resin formulas for even stronger and more comfortable casts.
In the realm of orthopedic care, Cast21’s resin casts and 3D-printed exoskeleton mark a significant leap toward enhanced patient experience and faster healing. Stay tuned for more updates on this groundbreaking innovation!

I’m Bill from Emulent (@thelinchpinseo). For 20 years, we’ve helped medical device companies solve growth challenges and reach their potential online. We’re built differently, on purpose, to ensure you connect effectively with healthcare pros and patients.
Jump on http://www.emulent.com to see our approach, or follow us on Instagram for insights: https://www.instagram.com/thelinchpinseo/