Polymer Technology Revolutionizing Diabetes Care
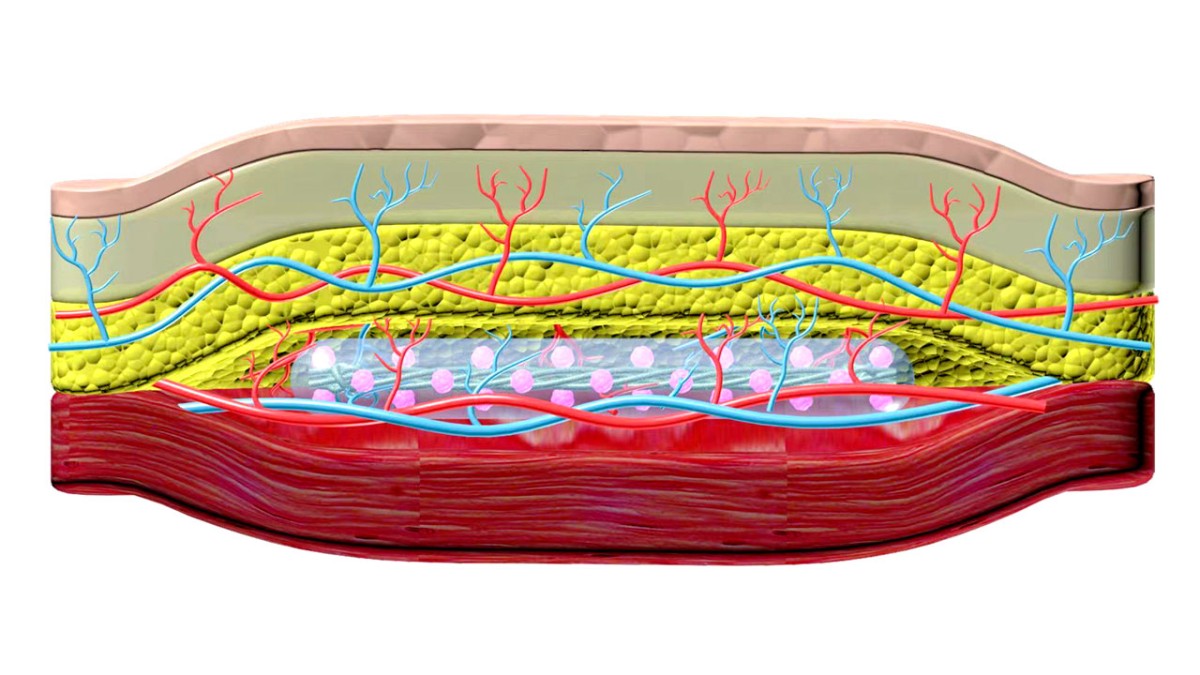
In a groundbreaking collaboration, researchers from Cornell and the University of Alberta, Edmonton, have pioneered a novel technique for treating Type 1 diabetes. Their innovation involves implanting a revolutionary device, a removable polymer thread, beneath the skin. This device can seamlessly secrete insulin, offering a game-changing alternative to traditional insulin injections and transplants, eliminating the need for immunosuppression.
Published in Nature Biomedical Engineering on December 5, the research paper titled “Inflammation-Induced Neovascularization of the Subcutaneous Tissue for the Long-Term Survival of Encapsulated Islets Without Immunosuppression” outlines a transformative approach co-developed by lead researchers Long-Hai Wang and Braulio A. Marfil-Garza.
Type 1 diabetes, characterized by the immune system’s attack on insulin-producing pancreatic cell clusters, leaves the body unable to regulate glucose entry into cells. Current treatments involve daily insulin injections or insulin pumps. However, Minglin Ma, a professor of biological and environmental engineering at Cornell, aimed to revolutionize diabetes control, particularly for children diagnosed with Type 1.

Minglin Ma, professor of biological and environmental engineering in the College of Agriculture and Life Sciences (CALS)
In 2017, Ma unveiled TRAFFIC (Thread-Reinforced Alginate Fiber For Islets enCapsulation), a removable polymer thread containing thousands of islet cells. This thread, implanted in the abdomen, acted as a micro-porous cage, allowing islets to secrete insulin in response to rising blood sugar levels. A more robust version created in 2021 demonstrated effectiveness in controlling blood sugar levels for up to six months in diabetic mice.
Collaborating with James Shapiro, a leader in islet transplantation from the University of Alberta, Ma merged their innovative strategies, resulting in SHEATH (Subcutaneous Host-Enabled Alginate THread). This two-step installation process involves inserting nylon catheters under the skin for four to six weeks, allowing blood vessels to form. Subsequently, islet devices are placed into the pocket created by the catheters, ensuring an intact vascular system.
The advantage of this technique lies in its minimal invasiveness, offering an outpatient procedure under local anesthesia. The researchers, including co-authors Ashim Datta and Dr. James Flanders, foresee challenges for long-term clinical application but express optimism in overcoming them. Persista Bio, a Cornell spinoff formed by Ma and collaborator Linda Tempelman, aims to address these challenges and develop a separate device to supply additional oxygen to the cells.
Supported by institutions like the National Institutes of Health, Novo Nordisk Company, Juvenile Diabetes Research Foundation, Hartwell Foundation, and the Diabetes Research Institute Foundation of Canada, this revolutionary polymer-based approach holds promise for transforming diabetes management on a global scale.

Do they respond to fructose? What splits the sucrose?
Is response fast/big enough to manage a scoop of ice cream, or a candy bar?